how to read an rf factor in chromatography In thin-layer chromatography, the retention factor (Rf) is used to compare and help identify compounds. The Rf value of a compound is equal to the distance traveled by the compound . The official source for NFL news, video highlights, fantasy football, game-day .
0 · why rf value not good
1 · why is rf importnat chemistry
2 · why are rf values useful
3 · what is rf value chromatography
4 · what does rf value mean
5 · rf value in paper chromatography
6 · how to measure rf values
7 · how to calculate rf values
• Anticollision: multiple cards may be selected and managed in sequence . NXP .
why rf value not good
It is possible to determine the characteristic rate of movement of each substance on the chromatography paper as the moving phase moves at a certain temperature and for a specific solvent. This is represented by the R f value, which stands for relative front or retardation factor.
why is rf importnat chemistry
A convenient way for chemists to report the results of a TLC plate in lab notebooks is through a "retention factor",\(^2\) or \(R_f\) value, which quantitates a compound's movement (Equation \ref{2}). In this article we will learn about Rf value also known as Retention Factor. Here we will discuss calculation of Rf values and its importance in thin layer chromatography. Key .
Learn about the Rf value, a key parameter in chromatography that allows for the identification and analysis of individual components in a mixture. Discover why the Rf value is important, how it's .In thin-layer chromatography, the retention factor (Rf) is used to compare and help identify compounds. The Rf value of a compound is equal to the distance traveled by the compound . Retention Factor. After a separation is complete, individual compounds appear as spots separated vertically. Each spot has a retention factor (Rf) which is equal to the distance migrated over the total distance covered by .
In planar chromatography in particular, the retardation factor R F is defined as the ratio of the distance traveled by the center of a spot to the distance traveled by the solvent front. [2] .
It is possible to determine the characteristic rate of movement of each substance on the chromatography paper as the moving phase moves at a certain temperature and for a specific solvent. This is represented by the R f value, which stands for relative front or retardation factor.A convenient way for chemists to report the results of a TLC plate in lab notebooks is through a "retention factor",\(^2\) or \(R_f\) value, which quantitates a compound's movement (Equation \ref{2}).
why are rf values useful
what is rf value chromatography
contactless card protection wallet uk
In this article we will learn about Rf value also known as Retention Factor. Here we will discuss calculation of Rf values and its importance in thin layer chromatography. Key words: Rf value or Retention factor, Thin layer chromatography (TLC), .
In thin layer chromatography, retention factor (Rf) is the distance that a compound travels through the stationary phase (TLC plate) between the origin spot and the distance the solvent front moved above the origin.
Learn about the Rf value, a key parameter in chromatography that allows for the identification and analysis of individual components in a mixture. Discover why the Rf value is important, how it's calculated, and the factors that influence it.In thin-layer chromatography, the retention factor (Rf) is used to compare and help identify compounds. The Rf value of a compound is equal to the distance traveled by the compound divided by the distance traveled by the solvent front (both measured from the origin).
Retention Factor. After a separation is complete, individual compounds appear as spots separated vertically. Each spot has a retention factor (Rf) which is equal to the distance migrated over the total distance covered by the solvent. The \( R_f\) formula is \[ R_f= \dfrac{\text{distance traveled by sample}}{\text{distance traveled by solvent}} \]In planar chromatography in particular, the retardation factor R F is defined as the ratio of the distance traveled by the center of a spot to the distance traveled by the solvent front. [2] Ideally, the values for R F are equivalent to the R values used in column chromatography. [2]
Rf values, or the Retention Factor, is a ratio used to describe the relationship between the distance moved by components in a mixture relative to the distance moved by the solvent. It is calculated by dividing the distance moved by the component . Learn how to calculate and interpret RF values in chromatography with our comprehensive educational guide. Master the principles of RF values and their significance in analytical chemistry.It is possible to determine the characteristic rate of movement of each substance on the chromatography paper as the moving phase moves at a certain temperature and for a specific solvent. This is represented by the R f value, which stands for relative front or retardation factor.
A convenient way for chemists to report the results of a TLC plate in lab notebooks is through a "retention factor",\(^2\) or \(R_f\) value, which quantitates a compound's movement (Equation \ref{2}). In this article we will learn about Rf value also known as Retention Factor. Here we will discuss calculation of Rf values and its importance in thin layer chromatography. Key words: Rf value or Retention factor, Thin layer chromatography (TLC), . In thin layer chromatography, retention factor (Rf) is the distance that a compound travels through the stationary phase (TLC plate) between the origin spot and the distance the solvent front moved above the origin.
Learn about the Rf value, a key parameter in chromatography that allows for the identification and analysis of individual components in a mixture. Discover why the Rf value is important, how it's calculated, and the factors that influence it.In thin-layer chromatography, the retention factor (Rf) is used to compare and help identify compounds. The Rf value of a compound is equal to the distance traveled by the compound divided by the distance traveled by the solvent front (both measured from the origin). Retention Factor. After a separation is complete, individual compounds appear as spots separated vertically. Each spot has a retention factor (Rf) which is equal to the distance migrated over the total distance covered by the solvent. The \( R_f\) formula is \[ R_f= \dfrac{\text{distance traveled by sample}}{\text{distance traveled by solvent}} \]
In planar chromatography in particular, the retardation factor R F is defined as the ratio of the distance traveled by the center of a spot to the distance traveled by the solvent front. [2] Ideally, the values for R F are equivalent to the R values used in column chromatography. [2]
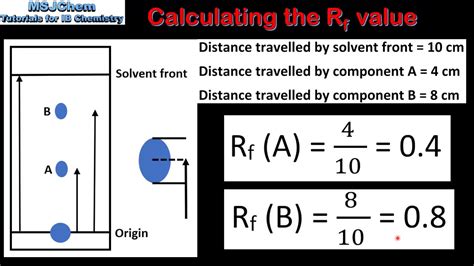
Rf values, or the Retention Factor, is a ratio used to describe the relationship between the distance moved by components in a mixture relative to the distance moved by the solvent. It is calculated by dividing the distance moved by the component .
what does rf value mean
rf value in paper chromatography
Most of the time these NFC cards are using encryption so it is not possible to emulate them .
how to read an rf factor in chromatography|why is rf importnat chemistry